GMP Audit Checklist
Maintain compliance and product quality with our comprehensive Good Manufacturing Practices (GMP) Audit Checklist. This free PDF template is designed to help you systematically assess and verify adherence to GMP standards within your manufacturing facility. By conducting regular GMP audits, businesses can ensure the safety, quality, and consistency of their products while complying with regulatory requirements.
Digitize this Checklist on Safetymint
- Create unlimited, customized checklists
- Add Actions, with automated reminders
- Works seamlessly with or without internet
- Access via web browsers, mobile or tablets

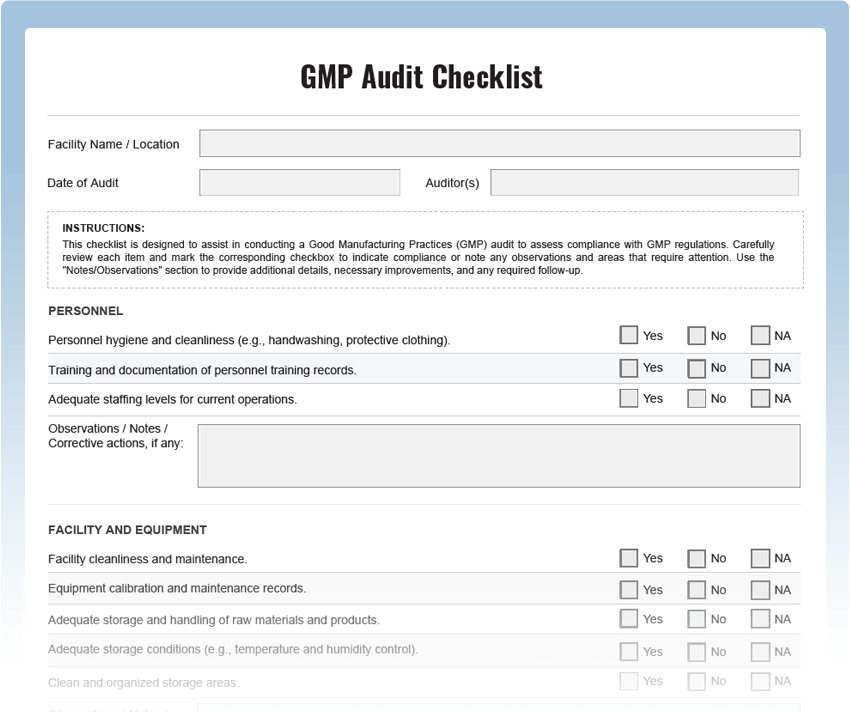
What is a GMP Audit Checklist?
A GMP Audit Checklist is a systematic tool used to evaluate and verify adherence to Good Manufacturing Practices within a manufacturing facility. It includes a series of checkpoints designed to assess various aspects of manufacturing processes, quality control, cleanliness, and documentation. Regular GMP audits are essential for ensuring that products are manufactured under conditions that meet regulatory standards and customer expectations.
Key Areas to Inspect in a GMP Audit Checklist:
- Facility Cleanliness: Assess the cleanliness and sanitation of the manufacturing facility.
- Personnel Hygiene: Evaluate employee practices related to personal hygiene and cleanliness.
- Raw Material Handling: Verify the proper storage and handling of raw materials.
- Quality Control: Review quality control measures and procedures in place.
- Documentation: Ensure that accurate and complete records are maintained.
- Equipment Maintenance: Inspect the maintenance and calibration of manufacturing equipment.
- Batch Record Review: Examine batch records for accuracy and compliance.
- Product Labeling and Packaging: Confirm that labeling and packaging meet regulatory requirements.
- Storage and Distribution: Assess storage conditions and product distribution practices.
- Complaint Handling: Review procedures for handling customer complaints and product recalls.
Benefits of Using a GMP Audit Checklist:
- Compliance: Ensure compliance with regulatory requirements and industry standards.
- Product Quality: Maintain consistent product quality and safety.
- Customer Confidence: Build customer trust through adherence to GMP standards.
- Risk Mitigation: Identify and mitigate risks associated with non-compliance.
- Continuous Improvement: Use audit findings to drive improvements in manufacturing processes.
GMP Audit Checklist Best Practices:
- Regular Audits: Conduct GMP audits at predefined intervals or after significant process changes.
- Competent Auditors: Ensure that auditors are knowledgeable about GMP standards and regulations.
- Documentation: Keep detailed records of audit findings, corrective actions, and follow-ups.
- Corrective Actions: Address identified deficiencies promptly and track corrective actions to closure.
- Training: Train employees on GMP requirements and the importance of compliance.