GMP Housekeeping Checklist
Maintain impeccable hygiene and compliance with Good Manufacturing Practices (GMP) using our comprehensive GMP Housekeeping Checklist. This free PDF template covers all critical aspects of a rigorous housekeeping inspection, empowering you to uphold the highest standards of cleanliness and organization in your facility.
Digitize this Checklist on Safetymint
- Create unlimited, customized checklists
- Add Actions, with automated reminders
- Works seamlessly with or without internet
- Access via web browsers, mobile or tablets

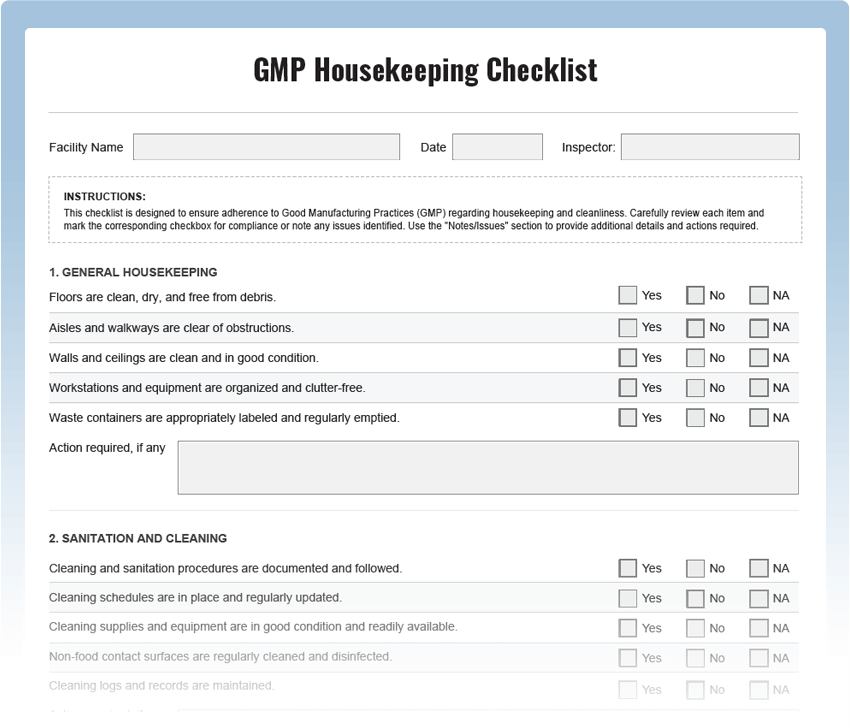
What is a GMP Housekeeping Checklist?
A GMP Housekeeping Checklist is a systematic tool used to assess the cleanliness and organization of a manufacturing facility. It includes a series of checkpoints designed to evaluate adherence to GMP regulations and best practices. Regular housekeeping inspections are essential to prevent contamination, ensure product quality, and meet regulatory requirements.
Key Areas to Inspect in GMP Housekeeping:
- Workstation Cleanliness: Check work areas for any spills, debris, or unclean surfaces that may compromise product integrity.
- Storage Conditions: Inspect storage areas to ensure proper segregation of raw materials, intermediates, and finished products to prevent cross-contamination.
- Labeling and Identification: Verify that all containers are appropriately labeled with clear identification of contents and expiration dates.
- Waste Management: Assess waste disposal procedures to ensure segregation of hazardous and non-hazardous waste.
- Sanitization Practices: Check if cleaning and sanitization procedures are followed diligently and at appropriate frequencies.
- Personal Hygiene: Ensure that personnel adhere to proper gowning, handwashing, and hygiene practices.
- Equipment Cleaning: Inspect manufacturing equipment to verify that cleaning procedures are in place and followed meticulously.
- Documentation Compliance: Review cleaning records and logbooks for accuracy and completeness.
Common GMP Housekeeping Hazards:
Inadequate housekeeping in a GMP environment can lead to various hazards, including:
- Cross-Contamination: Improper segregation of materials may lead to contamination of finished products.
- Product Defects: Unclean equipment and workspaces can compromise product quality and lead to defects.
- Regulatory Non-Compliance: Poor housekeeping practices can result in violations of GMP regulations.
- Safety Risks: Cluttered or unkempt areas can lead to workplace accidents and injuries.
GMP Housekeeping Best Practices:
- Regular Inspections: Conduct frequent housekeeping inspections to identify and address potential issues promptly.
- Training and Education: Provide comprehensive training to employees on GMP principles and housekeeping procedures.
- Cleaning Validation: Implement a robust cleaning validation program to ensure effective cleaning practices.
- Clear Procedures: Develop and communicate clear housekeeping protocols and expectations.
- Corrective Actions: Take immediate corrective actions to address any deficiencies found during inspections.